Oxidation Number Of Ions Worksheet Printable – Provide specific reasoning for the most common oxidation states of 3d elements. Are you looking for a worksheet on oxidation numbers of elements in compounds and ions for your chemistry students?this product includes 20 questions that ask students to. Do you need a worksheet to help gather information about student understanding of oxidation numbers, how to find them, and the rules around oxidation numbers?
Oxidation Number Worksheet 1Write The Rule Next To Your Answer
Oxidation Number Of Ions Worksheet Printable
This has a ½ full outer sublevel which is as stable as it can get since it cannot lose 8 electrons and be fe8+. The oxidation number of a monatomic ion equals the charge on the. A pure element has an oxidation number of 0.
Calculate Oxidation Numbers Practice Problems.
Rules for assigning oxidation numbers. Chemists have developed rules used to assign oxidation numbers. When arsenic is heated in oxygen,.
The Following Rules Will Help You Determine The Oxidation State Of An Atom Or Ion:
Do you need a worksheet to help gather information about student understanding of oxidation numbers, how to find them, and the rules around oxidation numbers? The oxidation number of an element in a monatomic ion equals the charge of the ion. (e.g., all group ia ions are +1;
All Group Iia Ions Are +2;
Oxidation numbers are real or hypothetical charges on atoms, assigned by. Assign oxidation numbersto each of the atoms in the following compounds: Give the oxidation numbers of all the elements in the following molecules and ions:
Therefore, The Sum Of The Positive Charges.
2 cr+ + sn4+ → 2 cr3+ + sn2+. Identify the oxidizing agent, reducing agent,. All the following ions have oxidation.
In This Oxidation Numbers And Ionic Compounds Worksheet, Students Write The Formula For Compounds Given Ions, They Write The Ions Given Formulas, They List Oxidation Numbers.
The oxidation number of any uncombined element is 0. Explain why fe3+ ion tends to form. One way of reflecting this is through changes in assigned oxidation numbers.
Oxidation States Of Elements In A Compound Provide Information About Size,.
A free atom has an oxidation. Identify the species being oxidised and reduced in each of the following reactions and write their half reactions: Oxidation numbers an oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction.
Determination Of Oxidation Number Or Valence Number Rules To Apply:
The net charges on all molecules is zero; What are the two most common.

Using Oxidation numbers to find formulas (polyatomic ions) worksheet

Assigning Oxidation Numbers Chemistry Tutorial YouTube

Oxidation Number Worksheet 1Write the rule next to your answer

Oxidation Numbers Worksheet Answers Thekidsworksheet

Assigning Oxidation Numbers Worksheet Answer Key Worksheet Resume

Solved 1. Oxidation numbers for the following compounds,

Oxidation States of Ions and Atoms

Atoms Vs. Ions Worksheet Name Date Period Atoms Vs Ions Fill

Assigning Oxidation Numbers Worksheet Fill Out and Sign Printable PDF

Assigning Oxidation Numbers Worksheet Answer Key Worksheet Resume

Oxidation Numbers Worksheet Answer Key worksheet

Table common ions with oxidation number
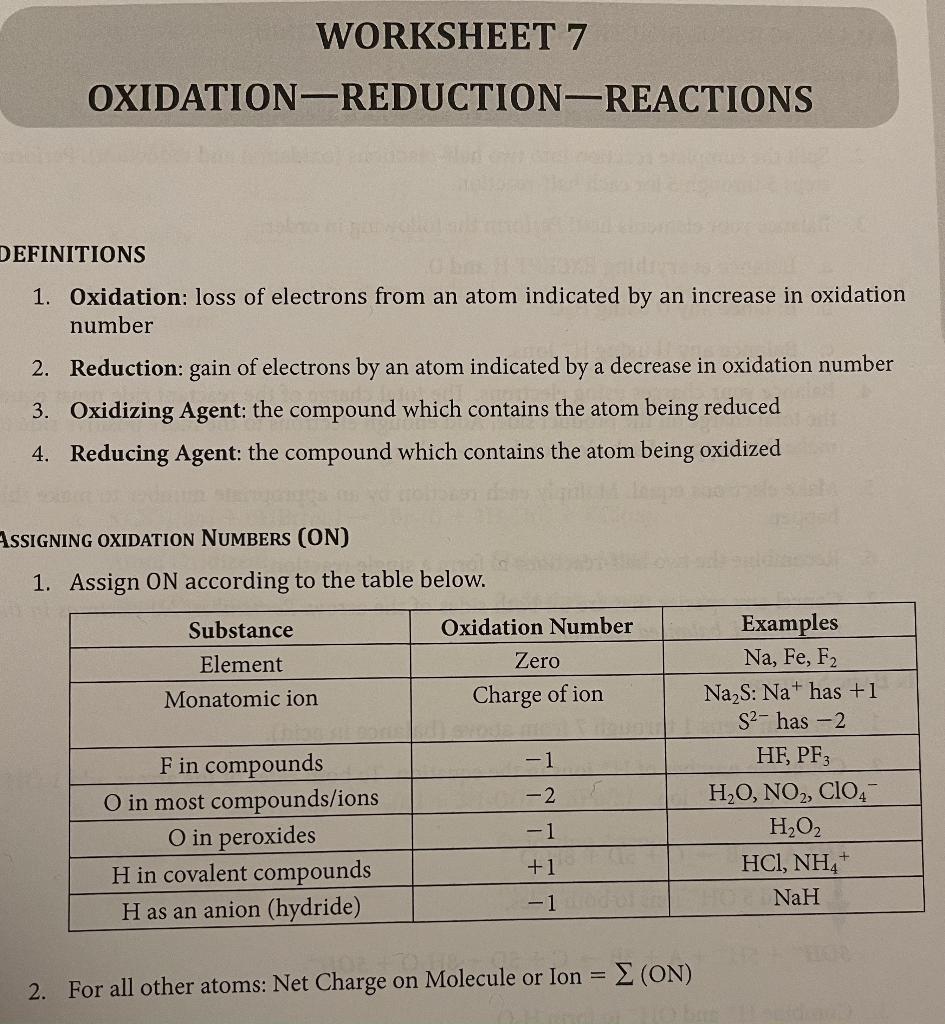
WORKSHEET 7 OXIDATIONREDUCTIONREACTIONS DEFINITIONS

Ionic compounds part 1 Predicted oxidation numbers YouTube

Oxidation Number Exercise printable pdf download